Search

News & Events
Major NHMRC grant prioritises strong skin for Aboriginal childrenEfforts to improve health outcomes for Aboriginal children have been accelerated thanks to almost $1 million in National Health and Medical Research Council (NHMRC) funds awarded to skin health researchers at The Kids Research Institute Australia.

News & Events
Funding boost for groundbreaking child health researchResearchers from The Kids Research Institute Australia will share in almost $4 million in grants to continue groundbreaking research to tackle childhood cancer, asthma, respiratory viral infections and more.

News & Events
Top-up funding announced to fast-track clinical trials of Spritz-OMResearchers developing a nasal therapy to prevent childhood ear infections and reduce overuse of antibiotics have received $300,000 in top-up funding.

News & Events
New partnership with Down Syndrome WAResearchers from the Wesfarmers Centre of Vaccines and Infectious Diseases, based at The Kids Research Institute Australia, are partnering with Down Syndrome WA to learn more about how respiratory syncytial virus, or RSV, affects children with increased medical vulnerability.

News & Events
The Kids Research Institute Australia researchers take out prestigious Premier’s Science AwardsThree outstanding researchers have won 2023 Premier’s Science Awards, with another inducted into the prestigious WA Science Hall of Fame.

News & Events
The Kids Research Institute Australia researchers awarded $11 million to support vital child health researchResearchers from The Kids Research Institute Australia have been awarded more than $11 million to support vital child health projects, under the Federal Government’s Medical Research Future Fund.

News & Events
Major grant awarded to tackle antibiotic resistanceVital research aiming to improve the treatment of potentially deadly Group A Streptococcus (Strep A) has been awarded $820,000 in the latest round of National Health and Medicine Research Council’s Ideas Grants.

News & Events
Landmark research investigating benefits of COVID-19 vaccination for kids set to launch at The Kids Research Institute AustraliaA first-of-its-kind national study looking at the optimal COVID-19 vaccination strategies for children and adolescents is set to begin at Perth’s The Kids Research Institute Australia thanks to a $3.8 million funding injection from the Australian Government’s Medical Research Future Fund (MRFF).
News & Events
Pneumococcal vaccine sees hospital admissions for deadly pneumonia slashed by halfThousands of children born in Papua New Guinea (PNG) no longer face a future cut short by severe pneumonia, thanks to the introduction of pneumococcal vaccination as part of the country’s National Immunisation Program.
News & Events
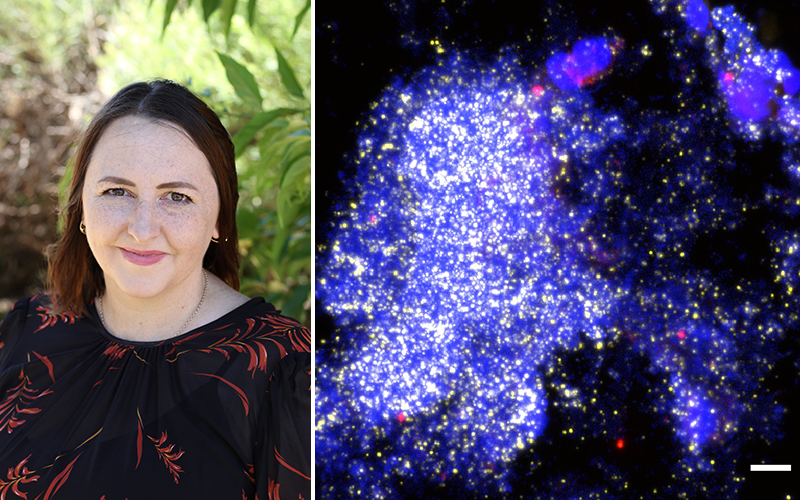
Bacterial slime causing persistent wet coughs for childrenResearchers using powerful microscopes have identified bacterial slime in the lungs of some children with persistent wet coughs.
