Search
Early 7vPCV schedules have limited impact on pneumococcal vaccine type carriage in PNG
Intervention to address young people's low levels of understanding, to promote their involvement in consent and reduce vaccination-related fear and anxiety.
Confirm the generalised IgE-trophic activity of the DTaP vaccine in pre-schoolers and demonstrate similar (albeit transient) effects in infants

Clinical Research Manager

Head, Infectious Disease Implementation Research

Co-head, Bacterial Respiratory Infectious Disease Group (BRIDG)
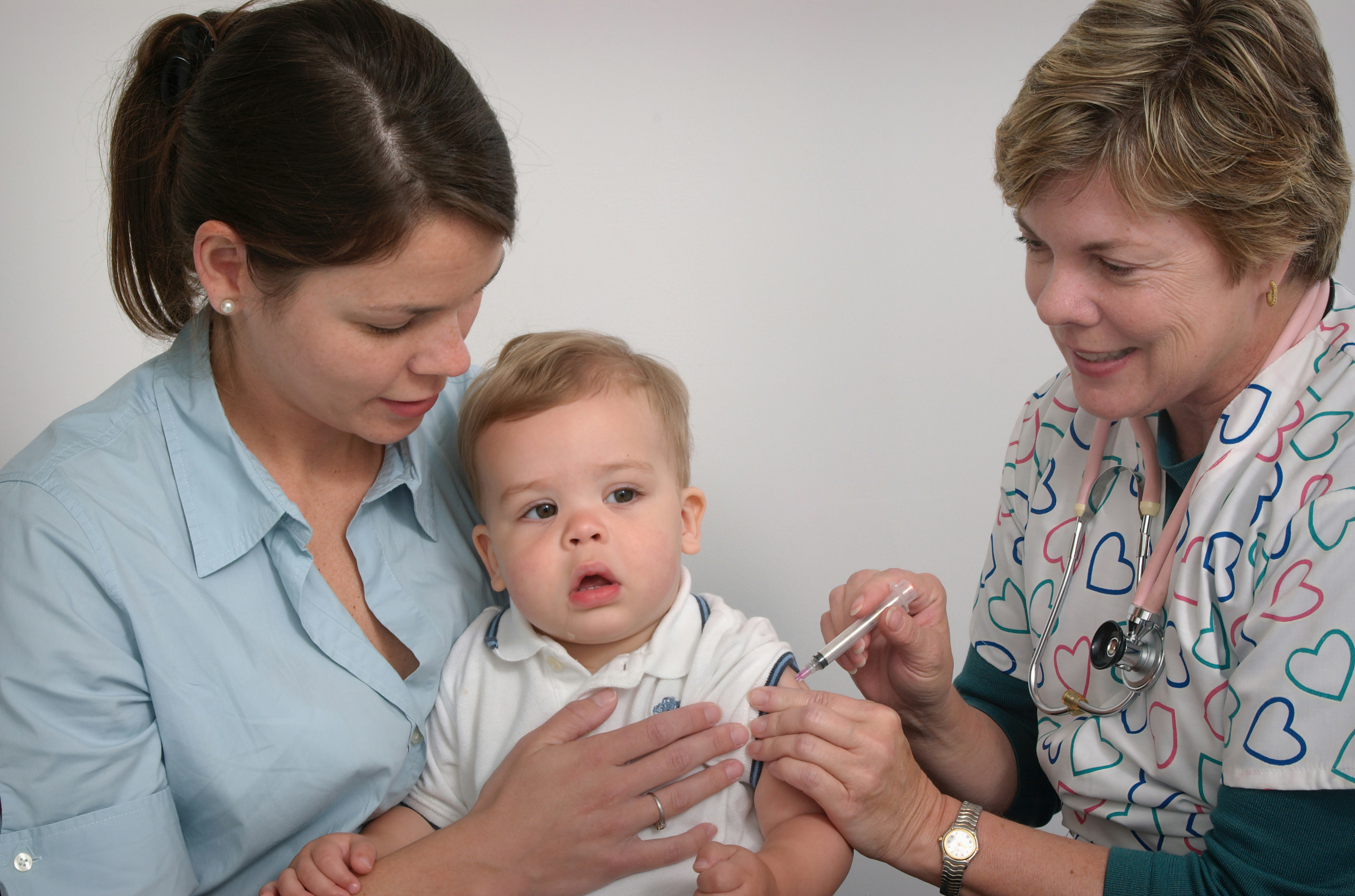
Researchers at The Kids Research Institute Australia have helped map the global impact of life saving vaccines to mark the 50-year anniversary of the Expanded Programme on Immunisation (EPI).

World-first immunisations providing protection against deadly respiratory syncytial virus (RSV) could be just months away thanks to global research efforts spanning multiple decades.

New collaborative research involving almost 600,000 pregnant mothers has demonstrated a dramatic increase in uptake of the whooping cough (pertussis) vaccine after identifying just 22 per cent of WA women had the maternal vaccination between 2012 – 2017.

Infectious disease researchers who used a decade of scientific evidence to advocate for a nationwide childhood influenza immunisation policy have earned a finalist position at the country’s most prestigious science awards – the Australian Museum Eureka Prizes.
