Search
Research
STopping Acute Rheumatic Fever Infections to Strengthen Health (STARFISH)STopping Acute Rheumatic Fever Infections to Strengthen Health (STARFISH) brings together a diverse and multidisciplinary research team to investigate the most effective environmental health initiatives (EHIs) aimed at reducing Strep A infections and prevent Acute Rheumatic Fever (ARF).
News & Events
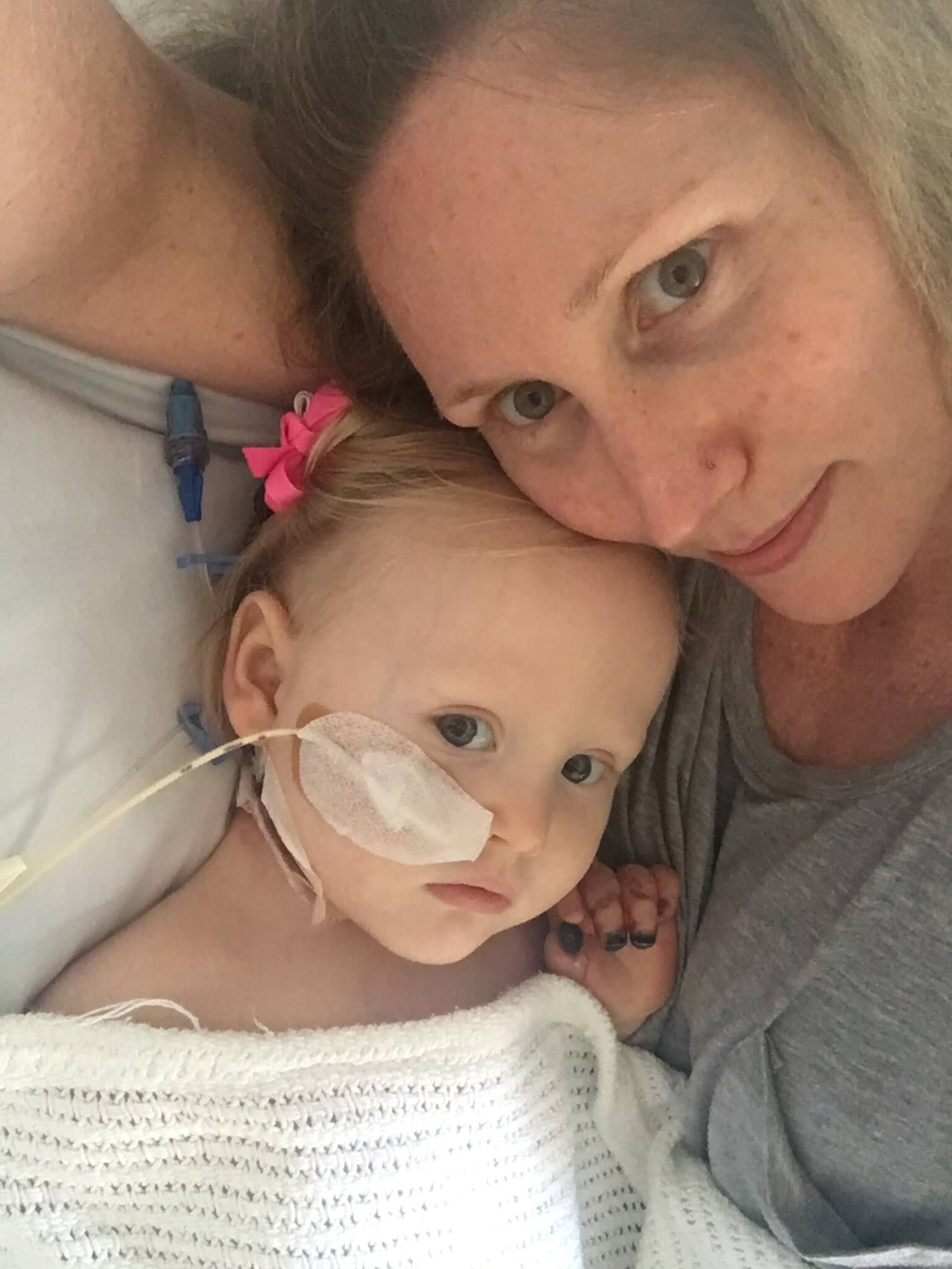
Isla's Invasive Strep A Story"I had never heard of invasive Streptococcus A disease before, and I was shocked to hear that it is actually three times more common than meningococcal disease and just as deadly yet there is no vaccine to protect against it."
Research
Host-dependent resistance of Group A Streptococcus to sulfamethoxazole mediated by a horizontally-acquired reduced folate transporterDescribed antimicrobial resistance mechanisms enable bacteria to avoid the direct effects of antibiotics and can be monitored by in vitro susceptibility testing and genetic methods. Here we describe a mechanism of sulfamethoxazole resistance that requires a host metabolite for activity.
Research
Standardization of Epidemiological Surveillance of Acute Poststreptococcal GlomerulonephritisAcute poststreptococcal glomerulonephritis (APSGN) is an immune complex-induced glomerulonephritis that develops as a sequela of streptococcal infections. This article provides guidelines for the surveillance of APSGN due to group A Streptococcus (Strep A). The primary objectives of APSGN surveillance are to monitor trends in age- and sex-specific incidence, describe the demographic and clinical characteristics of patients with APSGN, document accompanying risk factors, then monitor trends in frequency of complications, illness duration, hospitalization rates, and mortality.
Research
Burden of disease and barriers to comprehensive care for rheumatic heart disease in South Africa: an updated systematic review protocolRheumatic heart disease (RHD) is responsible for a significant burden of cardiovascular morbidity and mortality, and remains the most common cause of acquired heart disease among children and young adults in low-income and middle-income countries. Additionally, the global COVID-19 pandemic has forced the emergency restructuring of many health systems, which has had a broad impact on health in general, including cardiovascular disease.
Research
Standardization of Epidemiological Surveillance of Group A Streptococcal PharyngitisPharyngitis, more commonly known as sore throat, is caused by viral and/or bacterial infections. Group A Streptococcus (Strep A) is the most common bacterial cause of pharyngitis. Strep A pharyngitis is an acute, self-limiting disease but if undertreated can lead to suppurative complications, nonsuppurative poststreptococcal immune-mediated diseases, and toxigenic presentations.
Research
Transmission potential of Streptococcus pyogenes during a controlled human infection trial of pharyngitisControlled human infection (CHI) models can provide insights into transmission of pathogens such as Streptococcus pyogenes (Strep A). As part of the Controlled Human Infection with Penicillin for Streptococcus pyogenes (CHIPS) trial, we explored the potential for transmission among participants deliberately infected with the Strep A emm75 strain.
Research
Specificity of the Modified Jones CriteriaJonathan Carapetis AM AM MBBS FRACP FAFPHM PhD FAHMS Executive Director; Co-Head, Strep A Translation; Co-Founder of REACH 08 6319 1000 contact@
Latest news & events at the Wesfarmers Centre of Vaccines & Infectious Diseases.
Research
The potential global cost-effectiveness of prospective Strep A vaccines and associated implementation effortsGroup A Streptococcus causes a wide range of diseases from relatively mild infections including pharyngitis to more severe illnesses such as invasive diseases and rheumatic heart disease (RHD). Our aim is to estimate the cost-effectiveness of a hypothetical Strep A vaccine on multiple disease manifestations at the global-level.
