Search

Eight outstanding researchers from The Kids Research Institute Australia and the Institute-led Broome STEM Festival are finalists in the 2025 Premier’s Science Awards.

Western Australian kids will have access to a needle-free nasal flu vaccine for the first time in 2026 as part of a new initiative to boost vaccination rates against the life-threatening virus.

Papua New Guinean researcher Dr Lincoln Timinao has been awarded the 2025 Deborah Lehmann Research Award (DLRA) for his work aimed at investigating the burden of malaria in young children.
Wesfarmers Centre of Vaccines and Infectious Diseases researchers Dr Janessa Pickering and Dr August Mikucki travelled to Broome last week for the official launch of the long-awaited Missing Piece story books.

Free Family-Friendly Science Fun During National Science Week 2025. Get ready for an awesome adventure into the world of Science, Technology, Engineering and Mathematics!

National research led by the Wesfarmers Centre of Vaccines and Infectious Diseases, based at The Kids Research Institute Australia, has secured more than $3.4 million to assess the epidemiology of respiratory syncytial virus (RSV) throughout the country and optimise Australia’s immunisation strategy.

A new study underway at the Wesfarmers Centre of Vaccines and Infectious Diseases, based at The Kids Research Institute Australia, is deliberately infecting tonsils with Strep A in the laboratory to test a range of potential vaccine candidates.
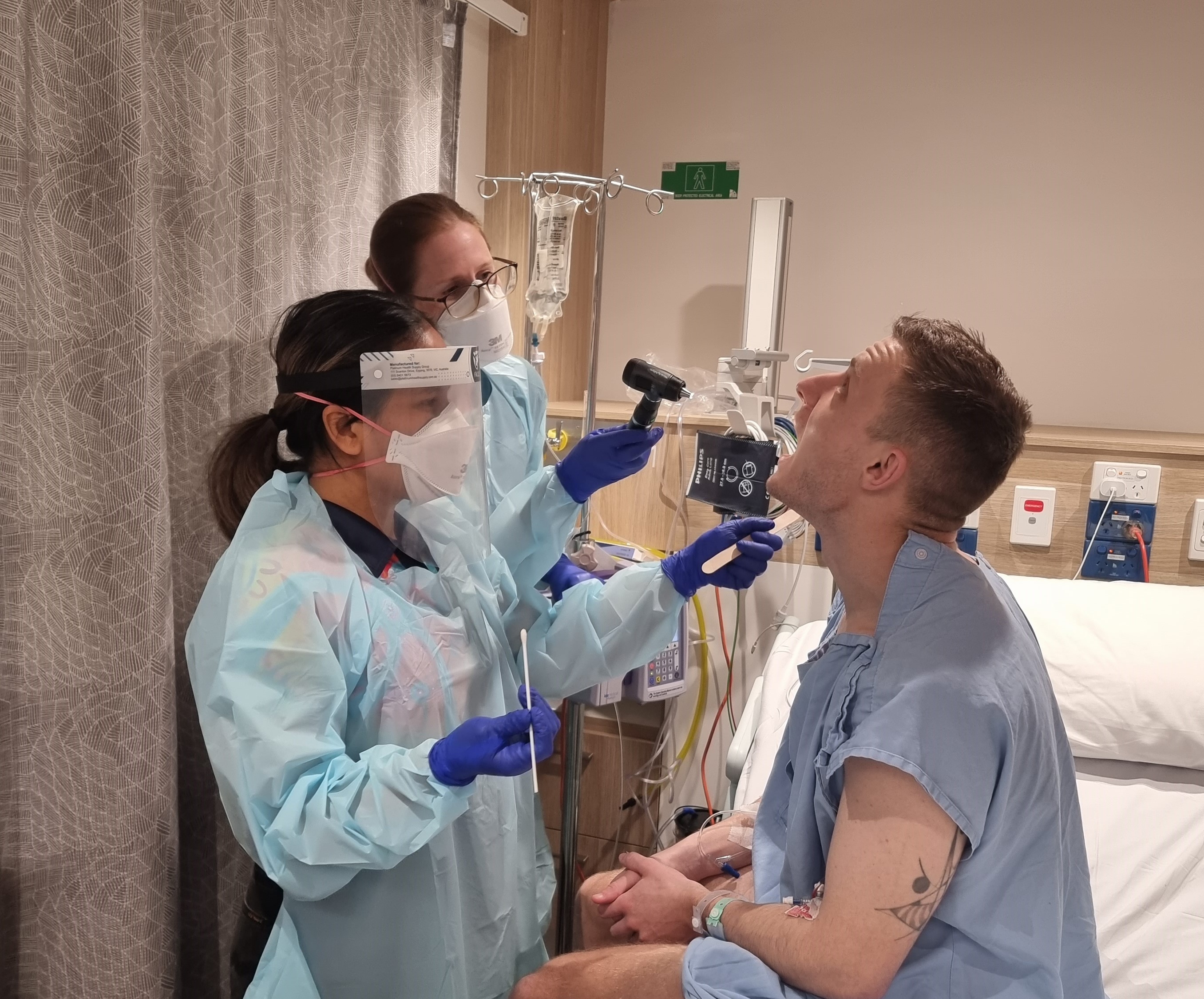
A unique study purposely giving participants Streptococcus pyogenes (Strep A) to learn how much penicillin it takes to prevent infection has found the amount needed is much lower than previously thought – a discovery that will transform thinking on treatment for people living with rheumatic heart disease (RHD).

The Wesfarmers Centre of Vaccines & Infectious Diseases brings together a number of independent researchers and research teams with a common aim; to find and deliver new and improved solutions to prevent and treat serious infections experienced by children or adolescents.
At the Wesfarmers Centre, we undertake research in five key areas of infections and immunisation to assist in children's health.
