Search

News & Events
New Co-directors for the Wesfarmers Centre of Vaccines and Infectious DiseasesDr Lea-Ann Kirkham and Dr Chris Blyth have been appointed as Co-Directors

News & Events
Rheumatic heart disease remains a major killer in Oceania regionA new study shows that people living in the Oceania region, including Australia, have the highest risk in the world of dying from rheumatic heart disease.
News & Events
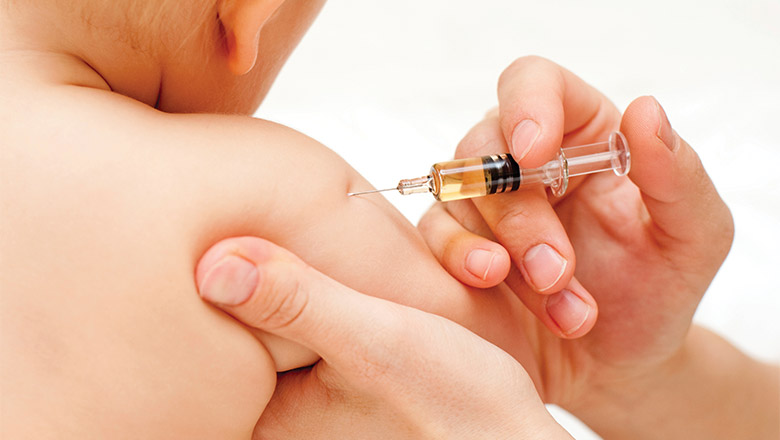
Extra whooping cough shot to protect your bubToddlers will now get an additional whooping cough vaccine to protect them against the potentially deadly disease.

News & Events
Taking on a common respiratory infection in kidsMapping when Respiratory Syncytal Virus (RSV) reaches its seasonal peak will assist how future vaccination programs are carried out.

News & Events
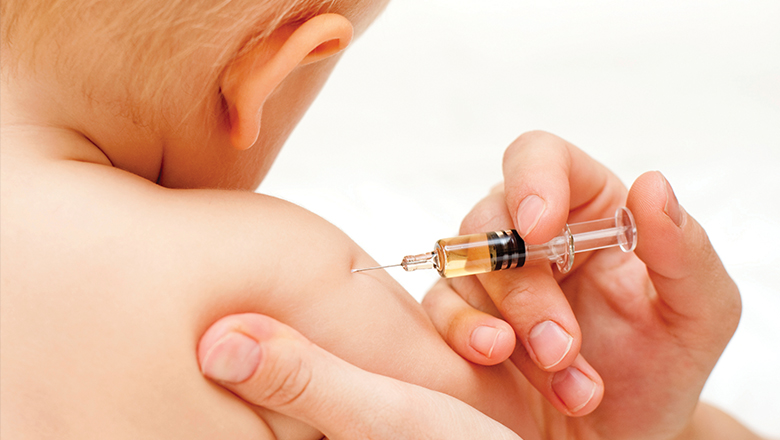
Extra whooping cough shots to protect bubsAn extra whooping cough vaccination for babies comes as a result of work by researchers at the Wesfarmers Centre of Vaccines and Infectious Diseases.
News & Events
Free vaccination public seminarImmunisation plays an important role in preventing disease within our community. Join us at a FREE public seminar on vaccinations.

News & Events
Vaccination Q&A with Dr Chris BlythImmunisation plays an important role in preventing disease within our community. Watch Dr Chris Blyth answer some commonly asked questions about vaccines.

News & Events
An open letter to WA familiesMy colleagues and I at Perth's The Kids Research Institute Australia study how to make current vaccines work better, reduce common side effects, and develop new vaccines.

News & Events
Vaccine trial aims to curb ‘superbug’Sarah Le Roi knows well how debilitating Clostridium difficile infection (CDI) can be. She was struck down with the 'superbug' while on holiday in the US.
News & Events
Wesfarmers Centre of Vaccine and Infectious Diseases Research Seminar Series 2014Wesfarmers Centre of Vaccine and Infectious Diseases Research Seminar Series 2014.Genetic and functional studies of leishmaniasis: understanding the role of HLA
